A few months ago, I realized I wanted to contribute towards finding a cure for Alzheimer’s disease (AD). To start, I fell back on one of my guiding principles as a scientist – that to find solutions to complex problems, one has to first understand the problem at a basic, fundamental level. In this blog post, I want to start from the basics and answer one seemingly simple question.
What is Alzheimer's disease?Searching for a definition for Alzheimer’s Disease
The majority of us know Alzheimer’s to be a neurodegenerative disease that causes gradual memory loss, speech problems and a loss of motor skills. A physician might provide a more sophisticated answer to my question.
Alzheimer’s is a neurodegenerative disease marked by a host of
disease phenotypes such as neuron death and the abnormal
accumulation of amyloid-beta plaques and neurofibrillary tangles.It turns out that this is the definition given by the National Institute of Aging for diagnoising AD and the definition that is generally accepted by the scientific community 1. Unfortunately, this is a definition with which I am unsatisfied.
Biological Roots of Alzheimer’s Disease
I am going to challenge our existing definition for diagnosing Alzheimer’s, but first I want to provide some historical background on Alzheimer’s that will motivate my challenge.
Alzheimer’s disease originated from a series of observations made in the last decade in which a certain class of patients seemed to die from a series of brain-related disease symptoms (loss of memory, decline in speech, cognitive impairment). Because the dominant disease symptoms in these patients were linked to cognitive function, a natural starting point for understanding this trend was to examine post-mortem brain-tissue samples to try and identify biological anomalies that did not occur in otherwise healthy brains.
Interestingly, there was a common thread among these patients – consistent biological artifacts that were present in disease brains, but not present in healthy brains! It turned out that disease brains exhibited much more neuron death than healthy brains. We eventually realized that disease brains to contain an overabundance of a certain peptide called amyloid-beta as well as neurofibrillary tangles (aggregates of hyperphosphorylated tau protein).
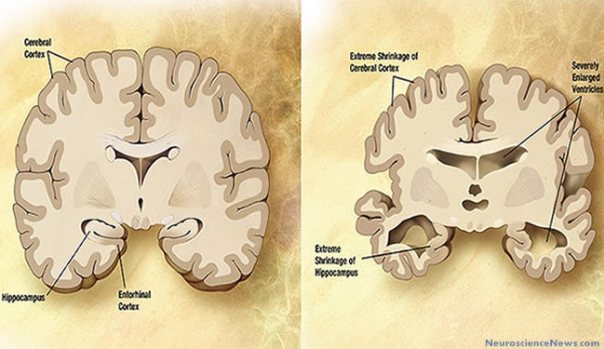 Image courtersy of http://neurosciencenews.com/
Image courtersy of http://neurosciencenews.com/
While the exact function of these two biological structures (amyloid-beta ad neurofibrillary tangles) is not know, we suspect they play a role in synaptic activity and modulating neuron signaling. In a future post, I will explain the technical details behind amyloid-beta and neurofibrillary tangles and their connection to Alzheimer’s disease. For now, it suffices to know that amyloid-beta and neurofibrillary tangles are abnormally abundant in the post-mortem brains of patients who exhibited serious brain-related disease symptoms. 2
Therefore when someone is diagnosed with Alzheimer’s disease, we are saying that this person has started to show some signs of cognitive decline and that if we could hypothetically dissect his/her brain we would expect to find abnormally large deposits of amyloid-beta and neurofibrillary tangles.
Reaching towards a more precise definition
Recall, the currently accepted definition of Alzheimer’s is given as follows
Alzheimer’s is a neurodegenerative disease marked by a host of
disease phenotypes such as neuron death and the abnormal
accumulation of amyloid-beta plaques and neurofibrillary tangles.Frankly, I find the above definition discomforting because it is imprecise. To illustrate, consider the following ambiguities that our working definition does not resolve.
- What defines memory loss?
- At what point, is memory loss severe enough to fall under the purview of Alzheimer’s disease?
- What defines abnormal accumulation of amyloid-beta plaques and neurofibrillary tangles?
I would been far more satisfied to hear a definition more along of the lines of the following.
A person is diagnosed with Alzheimer’s disease when the measure
amyloid-beta peptides exceeds X threshold, sparking neuron death
and disruption of cellular signaling pathways linked to
cognitive decline and memory loss.Notice the difference. The new definition resolves the underlying ambiguities by assigning a well-defined criterion (amyloid-beta exceeds X threshold) for diagnosing Alzheimer’s. In effect, diagnosing Alzheimer’s no longer invites a “maybe” response, but rather an explicit “yes / no” response.
So am I missing something here? Why have we not adopted more precision in our definition for Alzheimer’s?
Intractability of Precise Definitions
Tragically, our lack of precision reflects both a weakness in understanding the mechanisms of Alzheimer’s. Implicitly, this weakness in understanding also stems from the reality that the current state of biomedical technology simply cannot answer the questions needed to acquire a nuanced understanding of Alzheimer’s.
Let me explain. The crux of the ideal definition I put forward involves being able to assert a key phenomenon about Alzheimer’s – that past a certain threshold, amyloid-beta peptides causes Alzheimer’s. Unfortunately, the current state of research simply does not permit us to make any conclusive assertions required for this precise definition. We know amyloid-beta and neurofibrillary tangles are correlated with Alzheimer’s, but we have a hard time showing that there exists a causal relationship.
The difficulty of establishing a causal relationship immediately surfaces when I think reaching towards more precise definitions. For a complex disease like Alzheimer’s that is characterized by interdependent biological processes, I suspect the amount of data (brain-tissue samples, genomic sequences) needed to derive rigorous conclusions to even the simplest hypotheses involving amyloid-beta and neurofibrillary-tangles is prohibitively large. In other words, we will need massive breakthroughs in data-collection, statistical-processing and disease modeling to reach the description of Alzheimer’s that assuages my initial criticism.
Concluding Thoughts
In that vein, I arrive at a more refined perspective than the one with which I originally begun. My initial criticisms of imprecision are still valid and our present definitions leave me wanting more. Nevertheless, I acknowledge that I currently have no good solutions for addressing my criticisms and until we make some massive scientific breakthroughs, we will not arrive at that precise definition that I seek.